
In this compound, we have one atom of zinc, two atoms of nitrogen (one. For example, take the example of zinc nitrate, or Zn (NO 3) 2. For other compounds, this might get a little bit more complicated. Tricalcium phosphate would be entered as Ca3(PO4)2. The molar mass will be equal to: (1 atom x 56 grams/mole Fe) + (2 atoms x 35.5 grams/mole of chlorine) 127 grams/mole of iron (II) chloride. For example, calcium carbonate would be entered as CaCO3, not caco3. What is the molar mass of HCL 36.458 g/molHydrochloric acid / Molar mass.
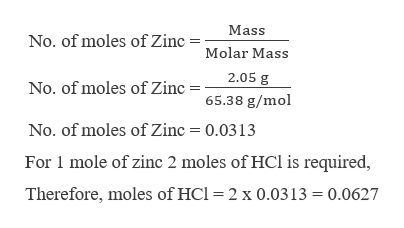
The chemical formula should be entered using standard format. Tris-hcl hydrochloride C4H13Cl2NO4562 - structure, chemical names, physical and chemical properties, classification, patents, literature, biological. The mass numbers of hydrogens isotopes are 1, 2, and 3, the most abundant being the mass 1 isotope generally called hydrogen (symbol H, or 1H) but also known as protium. This calculator is a convenient tool for calculating the molar mass of chemical compounds in lieu of using a periodic table. These include consumption of pH adjustment chemicals for RO feedwater, solubilities of scale forming compounds in reverse osmosis systems, and cation rejection calculations using charge balance (meq/l) in nanofiltration systems. 2Al(s)+ 6HCl(aq)-> 2AlCl3(s) + 3H2(g) Molar mass Al26.98 g/mol Molar mass HCl 36.45 Molar mass AlCl3 133.33 g/mol Molar mass H2 2.00g/mol If 2.5. Many other calculations require conversion into moles. Knowing the desired concentration of ClO2, the system integrator can calculate the consumption of each of the reactants using the stoichiometric relationship:ĢNaClO2 + NaOCl + 2HCl ↔ 2ClO2 + H2O + 3NaCl For example, certain types of chlorine dioxide (ClO2) generators would use sodium hypochlorite (NaOCl), sodium chlorite (NaClO2) and hydrochloric acid (HCl). Description, Pyridoxine HCl (Vitamin B6) is a form of vitamin B6. Molar mass of HCl 1 + 35.5 36.5 Number of moles of HCl mass / molar mass 85.6 / 36.5 2.345 moles Molarity number of moles / Volume 2.345 / 0.385 6.091 M How many moles are present. When calculating consumption of certain RO chemicals for reverse osmosis pretreatment or post-treatment, it is often necessary to convert into moles.


 0 kommentar(er)
0 kommentar(er)
